Our logistics team is responsible for delivering medicines to patients anywhere they may be. Every medicine is treated with extra care to ensure its safe and timely delivery.
Our logistics team is responsible for delivering medicines to patients anywhere they may be. Every medicine is treated with extra care to ensure its safe and timely delivery.
Resources
-
July 01, 2020Read more »
-
July 01, 2020Read more »
When our patients submit an inquiry for a medicine, they are then personally contacted by one of our Patient Support team members who assist them through the entire process of ordering, sourcing and delivering their medicines.

-
March 23, 2020Read more »
Here are some frequently asked questions and answers that touch on important COVID-19 related updates.
-
March 06, 2020Read more »
After submitting a request and receiving a quote from our Patient Support Team, patients often have many questions regarding the price that they receive. In this article, you can find a breakdown of the total cost of a medicine we are supporting you to access in addition to resources on how you can possibly get it reimbursed.

-
March 02, 2020Read more »
In order to help you expedite the request process with us, we put together a quick prescription guideline.

-
January 21, 2020Read more »
5 common questions about the supplement that may be useful for ALS, Parkinson's and Alzheimers.

-
October 30, 2019Read more »
New analysis of Biogen’s aducanumab shows reduction in clinical decline in Alzheimer’s disease.
-
October 24, 2019Read more »
As a pioneer of a completely new type of global service, we understand that trust, security and credibility are paramount–especially when paying for medicines which are essential and often the only treatment option available. There are an increasing number of scam websites and we ourselves have been the subject of one such scam. In this article we’ll give you tips on how to spot these fakers.

-
September 27, 2019Read more »
I’m lucky. It's been 4 years since the first MND symptoms, only my voice has been impacted.

-
September 10, 2019Read more »
Two and a half years after his diagnosis, David found a medicine with off-label application for MND.

-
September 02, 2019Read more »
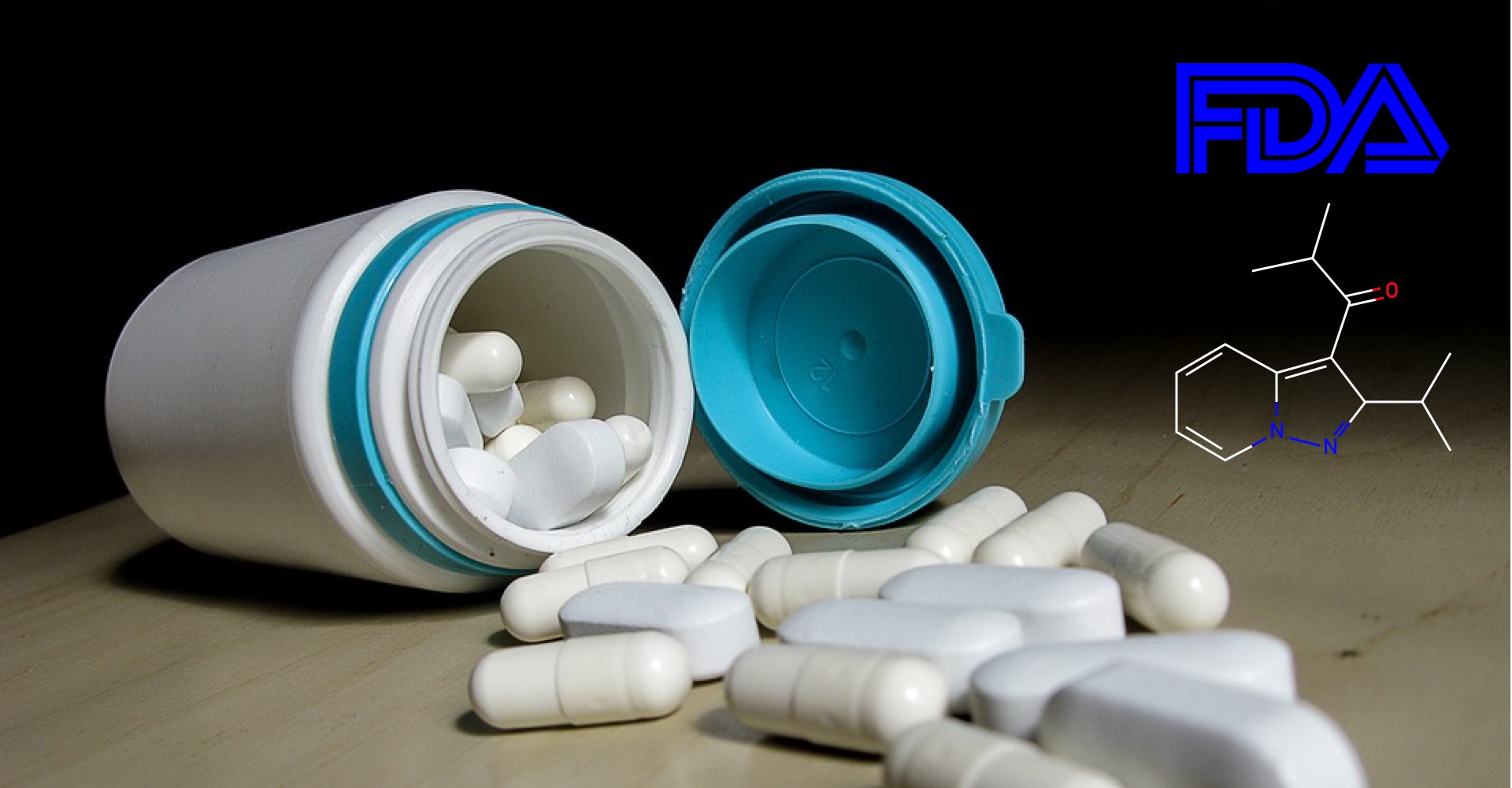
Kyowa Hakko Kirin's Parkinson's therapy, Nourianz (istradefylline), gains FDA approval as an add-on to levodopa/carbidopa.

-
August 29, 2019Read more »
Radicut/Radicava (edaravone), shown to slow the progression of ALS, is now approved for patients in China.

-
August 16, 2019Read more »
Orkambi and Symkevi have been rejected by the Scotland Medicines Consortium (SMC) due to their conclusion that the costs outweigh the benefits.

-
August 15, 2019Read more »
Vitrakvi (larotrectinib) recommended by the CHMP for approval in the EU.

-
August 14, 2019Read more »
 After 40 years with primary biliary cholangitis, Fiona's mother is still alive thanks to her daughter's dedication and a liver transplant.
After 40 years with primary biliary cholangitis, Fiona's mother is still alive thanks to her daughter's dedication and a liver transplant. -
July 29, 2019Read more »
After 3 years of living with ALS, Marc found a new medicine on the other side of the world.
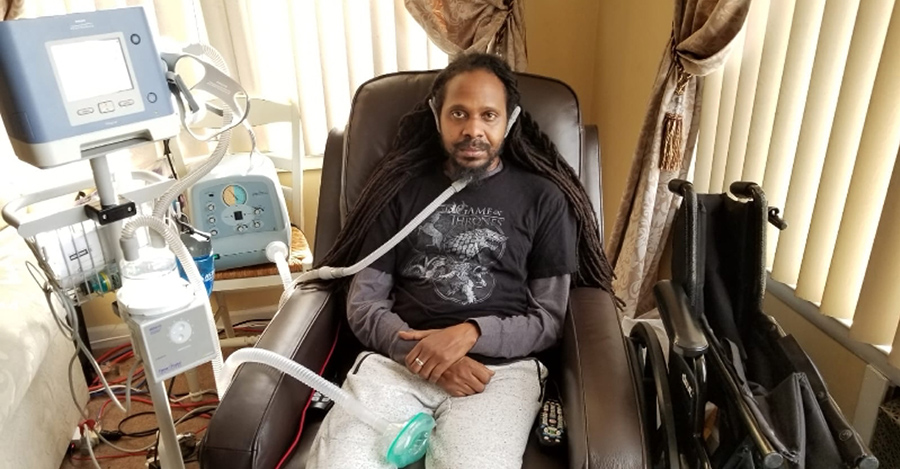
-
July 22, 2019Read more »
With tears, laughter and hope, Mark and his family find their own way to cope with ALS.

-
July 22, 2019Read more »
Diagnosed with ALS only 6 months ago, Peter went against the current to find more options to treat his disease.

-
Read more »
MN-166 (ibudilast) will be part of the Phase III clinical trial on progressive multiple sclerosis subjects with secondary progressive MS without relapses.

-
Read more »
New skin cancer medicine now approved in the EU for adults with metastatic or locally advanced cutaneous squamous cell carcinoma.

-
June 17, 2019Read more »
Still walking and talking after 4 years with ALS: we share Bakr's experience of living with a rare disease in the UAE to further his goal of creating awareness and to connect with other ALS patients.

-
June 14, 2019Read more »
Phase 2 clinical trial shows that ibudilast is linked to a 48% slowing in the progression of brain atrophy compared to placebo for patients with progressive MS.
-
Read more »
Vyndaqel (tafamidis) is now approved in the U.S. after study showed increased survivorship rate and reduced hospital time due to heart related problems.

-
April 24, 2019Read more »
Approval for next phase of clinical trials puts ALS patients one step closer to accessing new treatment shown to slow disease progression.
-
March 13, 2019Read more »
Downloadable fact sheet answering the most common question TheSocialMedwork asked by healthcare providers.

-
February 28, 2019Read more »
Based on promising results from phase III clinical trials, Health Canada has decided to extend the use of Kalydeco (ivacaftor) to include children aged 1 to 2 years.

-
February 28, 2019Read more »
In recognition of Rare Disease Day, our colleague, Sera, shares her story of her dad's fight with Multiple System Atrophy (MSA).
-
February 27, 2019
-
February 25, 2019Read more »
A recent clinical trial indicates that Symkevi/Symdeko (tezacaftor/ivacaftor) can safely and effectively loosen thick, sticky mucus for children 6-11 years old.

-
February 07, 2019Read more »
The new site-agnostic cancer medicine, Vitrakvi (larotrectinib), gets accelerated approval from the U.S. Food and Drug Administration (FDA).