
Newly approved in the EU: Durvalumab (Imfinzi) an immunotherapy for Stage III Lung Cancer
Lung cancer and bladder cancer are respectively the 1st and 10th most common types of cancers per incidence worldwide. Durvalumab (which has the brand name Imfinzi) is an immunotherapy that treats specific types of these two cancers. It is the first immunotherapy to demonstrate significant overall survival benefit in unresectable, stage III lung cancer; and it also provides an additional option for patients who have previously received treatment for advanced bladder cancer.
Durvalumab (Imfinzi) is a medicine used for treating locally advanced or metastatic bladder cancer (urothelial carcinoma), as well as unresectable* Stage III (locally advanced) Non-Small Cell Lung Cancer (NSCLC). It is the only immunotherapy approved for the treatment of this type of lung cancer, and the first to significantly reduce the risk of death in these patients.
* Unresectable cancers are those cancers which cannot be removed completely through surgery.
Durvalumab (Imfinzi) is an antibody indicated for the treatment of people with:
* Neoadjuvant treatments are administered before surgery with the aim of shrinking the tumor and increasing the chances of a successful surgery. Adjuvant treatments are additional cancer treatments given after the primary treatment in order to lower the risk that the cancer will come back. It may include chemotherapy, radiation therapy, hormone therapy, targeted therapy, or biological therapy.
The approval of durvalumab (Imfinzi) for unresectable Stage III (locally advanced) NSCLC was based on a study that involved a total of 713 patients whose disease had not progressed following chemotherapy and radiation. Patients were treated for up to 12 months.
For more details refer to the prescribing information at the bottom of the page.
Durvalumab (Imfinzi) was tested in a clinical trial which involved 182 patients with locally advanced or metastatic bladder cancer (urothelial carcinoma), whose cancer progressed on or after platinum-based chemotherapy. The clinical study is still ongoing but durvalumab (Imfinzi) has already been approved with accelerated approval based on the good preliminary results from this trial.


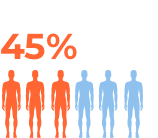
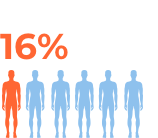
For more details refer to the prescribing information at the bottom of the page.
The most common side effects of durvalumab (Imfinzi) in people with bladder cancer (urothelial carcinoma) include: feeling tired, nausea, muscle or bone pain, swelling of the arms and legs, constipation, urinary tract infection, decreased appetite.
The most common side effects of durvalumab (Imfinzi) in people with NSCLC include: cough, upper respiratory tract infections, tiredness, shortness of breath, inflammation in the lungs (pneumonitis), and rashes.
Please note this is not intended to be a comprehensive guide. Consult your treating doctor and the prescribing information at the bottom of the page for full details of side effects.
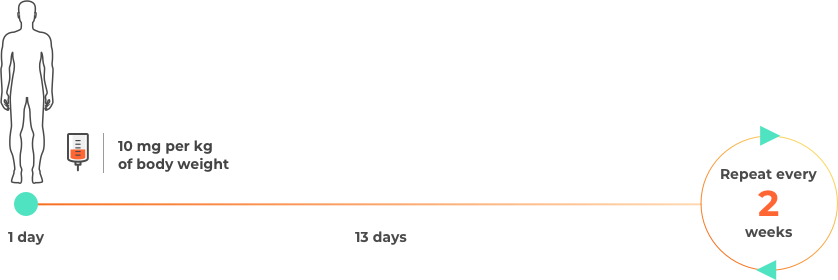
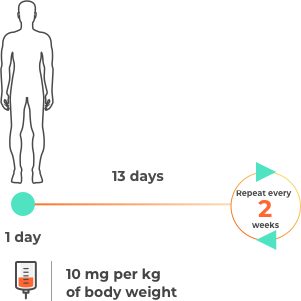
Please note this is not intended to be a treatment plan. For a personalised treatment plan, consult your doctor. For more details, you can also reference the full prescribing information at the bottom of the page.
Durvalumab (Imfinzi) is an immunotherapy, which means that it uses and boosts the body’s own immune system to try and fight the cancer.

For more details about the way durvalumab (Imfinzi) works, consult your doctor or see the prescribing information at the bottom of the page. This is not intended to be a comprehensive scientific explanation.

If you, or one of your relatives or friends are a patient with lung or bladder cancer, you might want to ask questions about the medicine for this disease. If you’d like to ask questions about how to get durvalumab (Imfinzi), or about our service in general, our team of qualified pharmacists and experts are ready to offer support and assist with queries. Our team speaks 17 different languages and is ready to help.
everyone.org makes the latest approved medicines available to patients, hospitals and doctors around the world, in a legally compliant way. We source innovative medicines at the best possible prices and ensure safe and efficient delivery. We have successfully delivered to over 88 countries worldwide, and have already supported over 11,000 doctors and patients.
Find out more